When it comes to synthesizing a peptide, the first thing that comes to mind is the number of stoichiometric equivalents to use. Sometimes that number is as few as 1.5, sometimes it’s as high as 20!
But have you ever thought about the liquid volume that contains those molecules and how that might affect the success of your coupling reaction? In this post I will discuss the impact of amino acid concentration in the overall success of solid phase peptide synthesis.
Nowadays, many of us are preparing peptides using automated peptide synthesizers. These systems typically come with default coupling methods and standardized amino acid concentrations. But what if the solution viscosity was not a concern? What if you had the freedom to manipulate amino acid concentration to more fully optimize your synthesis? What volume would you choose for dissolving your amino acids and does it even matter?
I have access to a Biotage® Initiator+ Alstra™ which, with its robotic liquid handler, enables me to accurately handle and transfer solutions without concern for viscosity and therefore address the above questions. I decided to synthesize two peptides, one composed of 10 amino acids (ACP65-74) and the second composed of 37 amino acids (GLP-11-37) using either a 0.2 M or 0.5 M amino acid solution concentration.
The 2.5 fold increase in concentration results in a 2.5 fold decrease in total solution volume required per coupling and a 1.5 fold increase of amino acid concentration in the final coupling solution. Although the differences in concentration seem modest, the results of the synthesis can be significantly different.
For the synthesis of ACP65-74 the difference in overall crude peptide quality was not significant for the two amino acid concentrations. This shouldn’t be too surprising though. Most short peptides can be synthesized in high yields and high crude purity without much synthesis optimization. This segment of ACP is known to be a difficult sequence to synthesize, but the use of DIC and Oxyma as the coupling reagents readily enables this synthesis in very high crude purity and yield.
The differences were much more dramatic, however, when I synthesized full length GLP-1. When synthesized with 0.2 M amino acid solutions, the desired peptide was the majority product but, there are clearly many other products present in the crude sample as well, Figure 1.
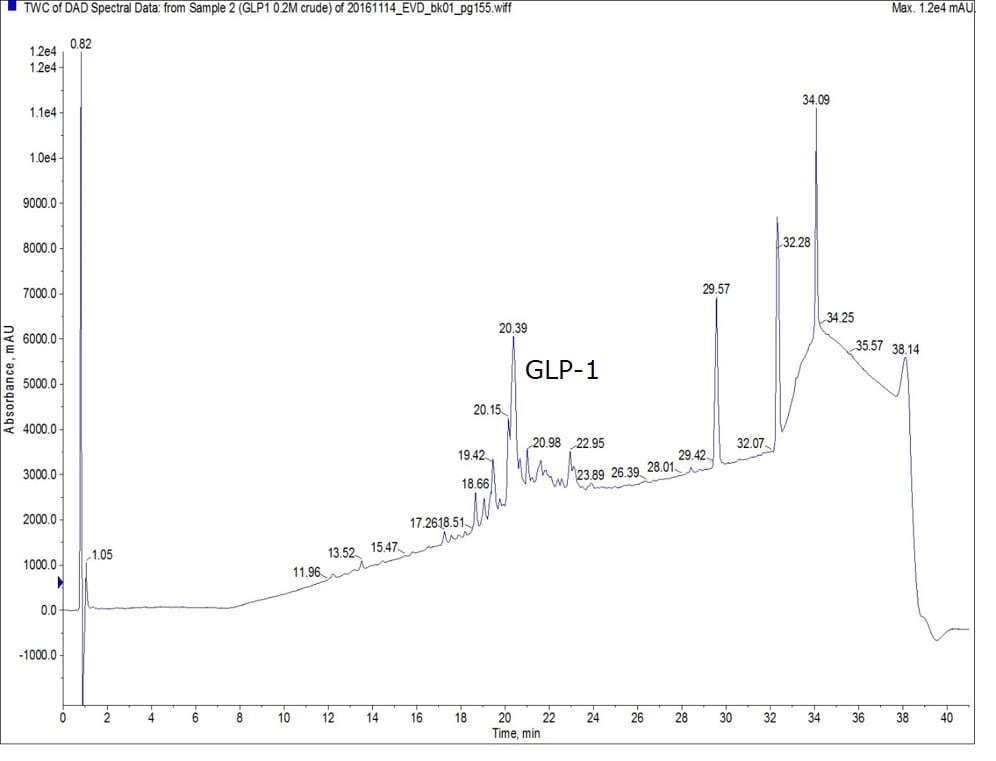 Figure 1: Crude analytical chromatogram of GLP-1 synthesized using 0.2M amino acid solution. Please note, the late eluting peaks are likely due to the presence of residual protecting groups in the solution as no peptidic character is present in the mass spectrum of these peaks.
Figure 1: Crude analytical chromatogram of GLP-1 synthesized using 0.2M amino acid solution. Please note, the late eluting peaks are likely due to the presence of residual protecting groups in the solution as no peptidic character is present in the mass spectrum of these peaks.
When the same peptide was synthesized using 0.5 M amino acid solutions (and the same protocol) the crude purity improved significantly, Figure 2. Interestingly, the doublet peak labeled GLP-1 is nearly baseline resolved, but the corresponding mass trace indicates both peaks contain GLP-1, suggesting a conformational distribution within the crude peptide solution.
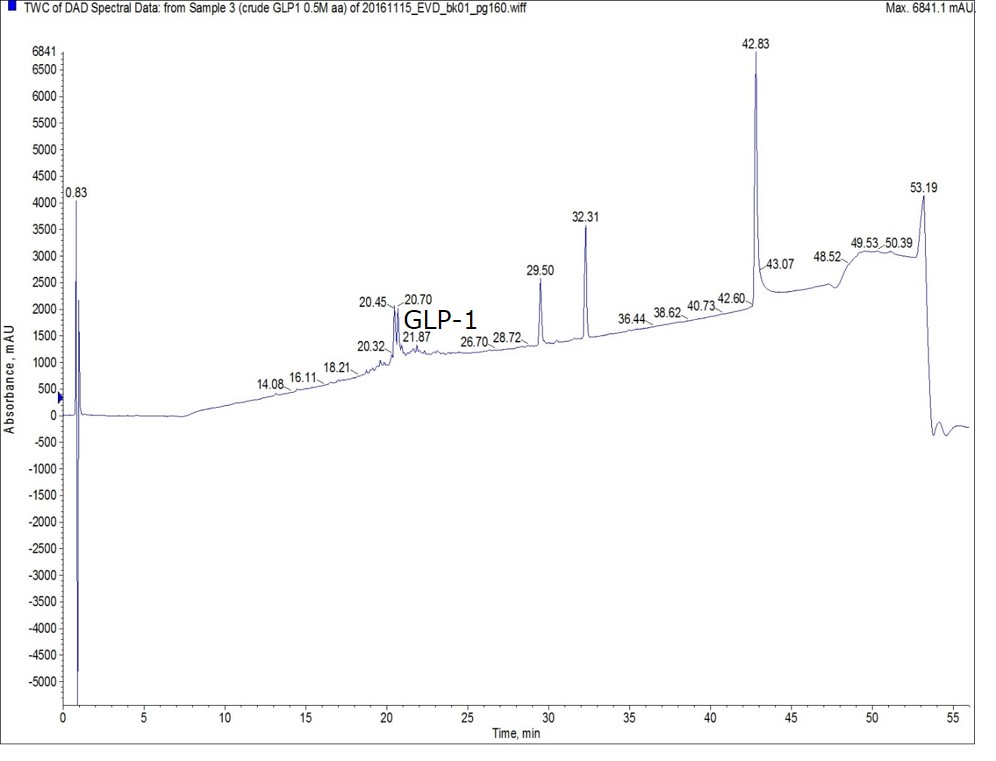
Figure 2: Crude analytical HPLC chromatogram for GLP-1 synthesized using 0.5 M amino acid solutions. Please note, the late eluting peaks are likely due to the presence of residual protecting group as no peptidic character is present in the mass spectrum of these peaks.
There is such a thing as too concentrated of solutions though, especially if you’re using heat to expedite your coupling reactions. One must be considerate of the viscosity of the resin slurry. Two complications can arise in this case. The first, and most obvious, is that without full suspension of the resin in the amino acid solution you can’t expect to achieve complete couplings. It’s all about accessibility…
But the second complication arises when microwave heating (or any heat source really) is applied. If the resin slurry is too thick (think fully wetted and suspended in the solution but with a consistency of molasses), then microwave irradiation can then be absorbed by the resin itself rather than the liquid solution. This results in hot spots, poor heat distribution throughout the sample, and poor peptide yields. I’ve even been so lucky as to have this result in resin overflowing out of my reaction vessel, Figure 3. Not a good day….

Figure 3: Resin overflowing from a reaction vessel due to high solution viscosity, causing in-vial overheating.
So to wrap things up, using more concentrated amino acid solutions can, in fact, improve your crude peptide purity, particularly for longer synthetic peptides. This results in higher total yield and less time spent at the HPLC purifying your peptide samples. Just remember though, you want your total liquid volume to be concentrated enough to improve your kinetic outcome, but dilute enough such that your resin is still free flowing in the liquid solution.
To learn more about how Biotage tools can improve your overall peptide workflow efficiency, follow the link below.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership